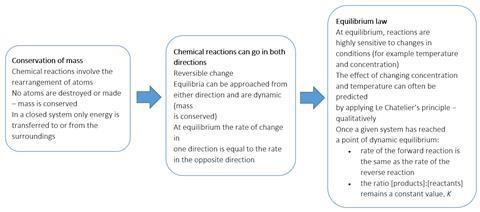
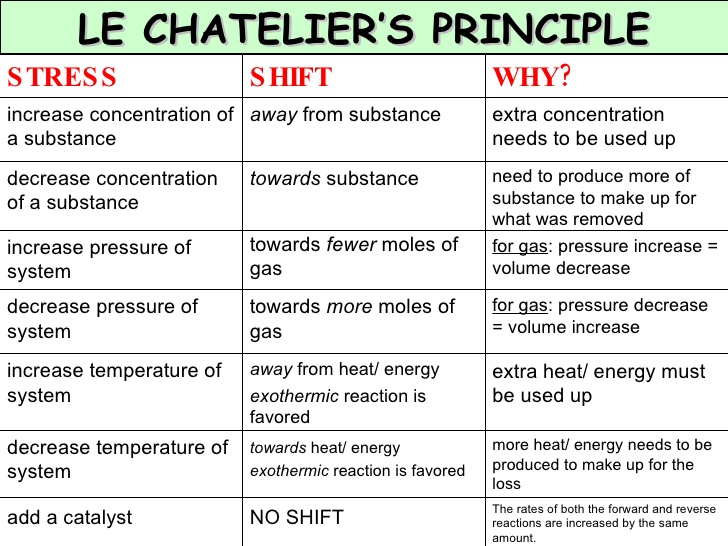
14/11/17 · Le Chatelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions (such as concentration, temperature and pressure changes) , the position of equilibrium shifts to counteract the change to reestablish an equilibrium If a chemical reaction is at equilibrium and experiences a change in pressure, temperature, or concentration of products orLe Châtelier's Principle Temperature Increasing temperature favors the production of brown NO 2 in the boiling water on the left The ice cold container on the right contains more molecules of colorless N 2 O 4 , so its color is lighterTo restore equilibrium, the system will favor a chemical pathway to reduce or eliminate the disturbance so as to restabilize at thermodynamic equilibriumPut another way, If a chemical system at equilibrium experiences a change in concentration, temperature or total pressure, the

Solved 5 Which Are Examples Of Equilibria Which Are Che Chegg Com
Temperature le chatelier's principle example
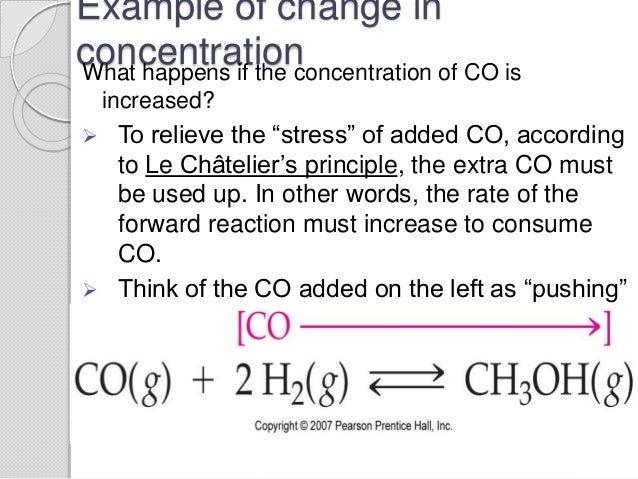
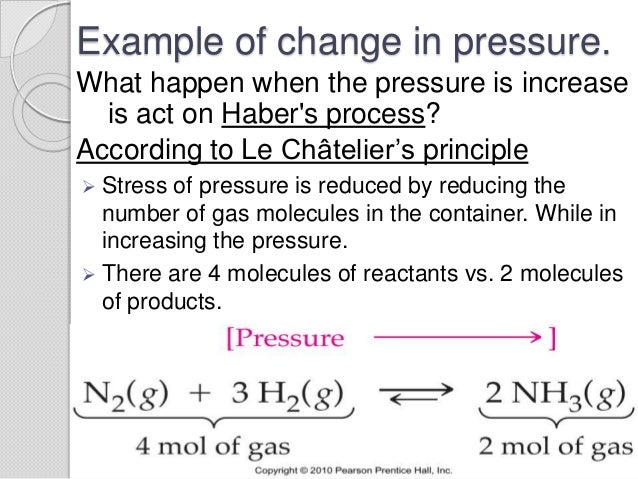
Temperature le chatelier's principle example-Using Le Châtelier's Principle Set up K eq = 393= (0387x)x (193x)3(0613x) Changes in Temperature The equilibrium "constant" is not constant with temperature Le Châtelier's Principle would suggest Qualitatively, if a reaction is endothermic then the equilibrium "constant" increases with temperatureLe Chatelier's Principle is important, because it allows us to shift an equilibrium to the side that we would like to favor For example the Haber Process produces ammonia reversibly #N_2(g) 3 H_2(g) > 2 NH_3(g)# The reaction is run at high pressures, because there are 2 moles of ammonia on the product side, but 4 moles of gas on the reactant side (3 mol of hydrogen and 1 mol of


Le Chatelier S Principle 2
UTRGV CHEM 1112 Section number COVER PAGE EXP # 6 Le Chatelier's principle And Buffers Experiment Number Name of Experiment CHEM L The University of Texas Rio Grande Valley Semester & Year *Student Mirthala Pruneda ID OBJECTIVE Concentration, temperature, the addition of strong base and strong acid to buffered and unbuffered, common ion effect INTRODUCTION Le Chatelier'sLe Chatelier's principle can be used to predict the behavior of a system due to changes in pressure, temperature, or concentration Le Chatelier's principle implies that the addition of heat to a reaction will favor the endothermic direction of a reaction as this reduces the amount of heat produced in the systemLe Chatelier's Principle states that a system always acts to oppose changes in chemical equilibrium;
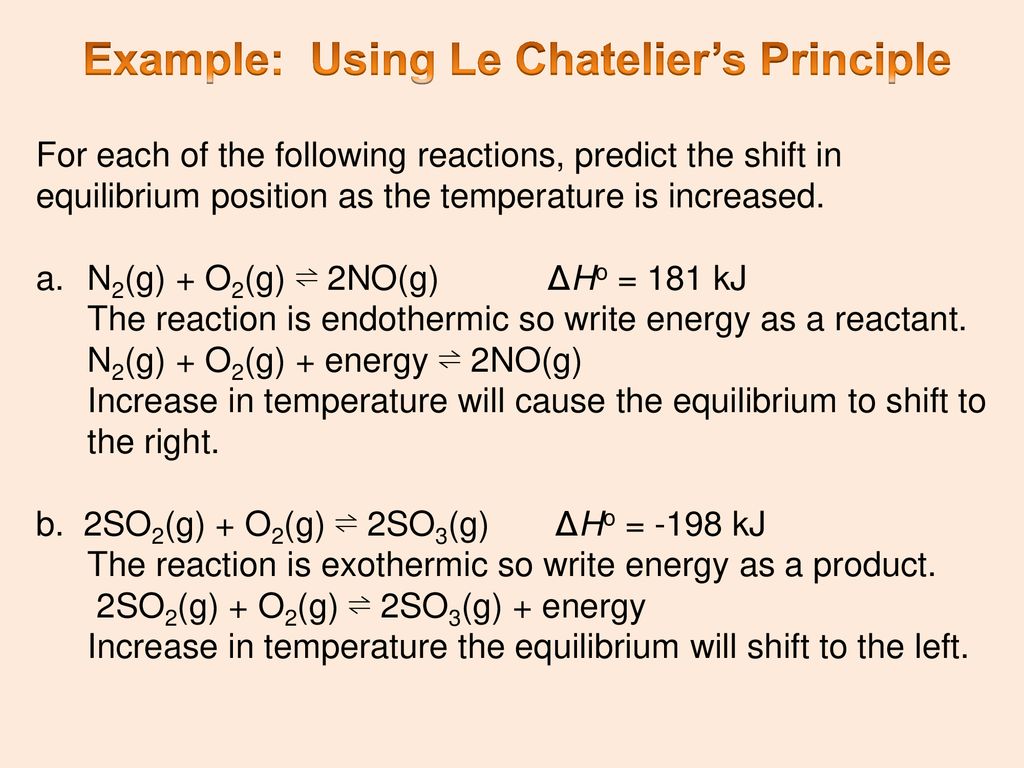
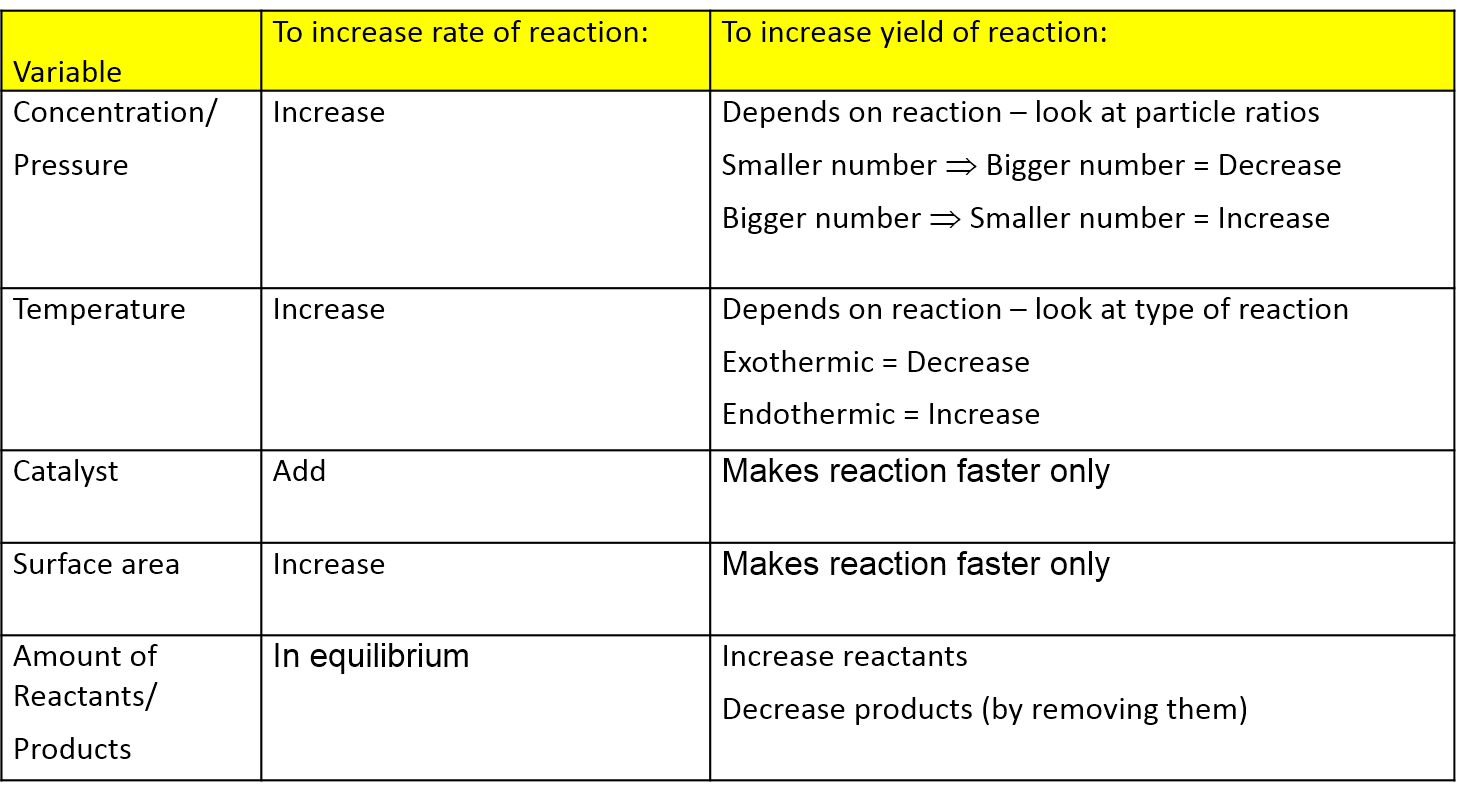
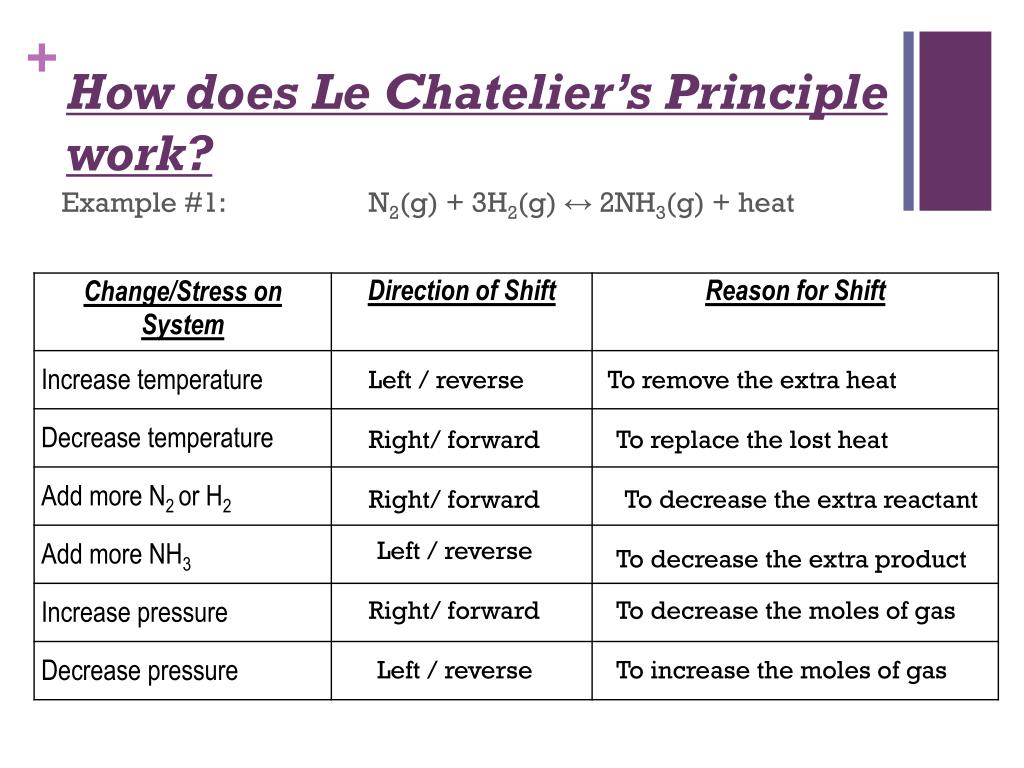
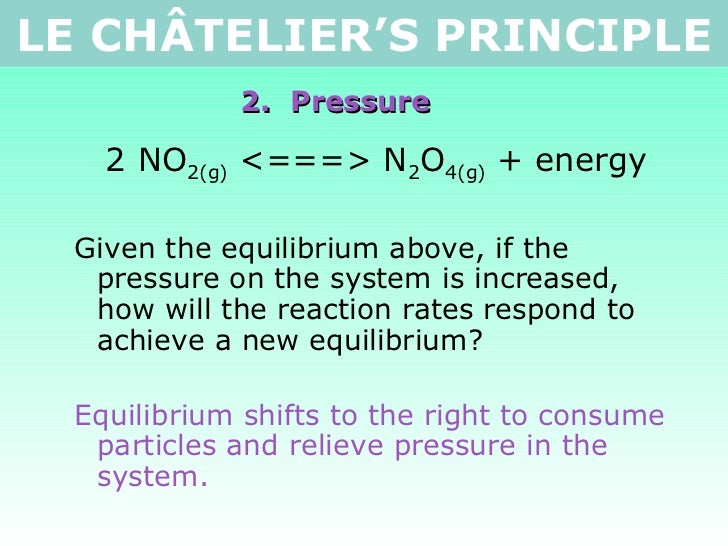
N 2 (g) 3 H 2 (g) ⇄ 2 NH 3 (g) Solution According to Le Chatelier's principle, if pressure is increased, then the equilibrium shifts to the side with the fewer number of moles of gasApplying Le Chatelier's Principle to Saturated Solutions Solubility tables like the one above, tell us the maximum mass of solute that will dissolve in a given mass of solvent under certain conditions Typically, the conditions are a temperature of 25 o C and a pressure of 1013 kPa (1 atm) Let's see what happens when we change either of these conditionsDr Shields discusses how to determine the direction the equilibrium will shift upon a temperature change based on the sign of the reaction enthalpy (H) Ge
Le Chatelier's principle can be used to predict the behavior of a system due to changes in pressure, temperature, or concentration Le Chatelier's principle implies that the addition of heat to a reaction will favor the endothermic direction of a reaction as this reduces the amount of heat produced in the systemAdd some product A ?In accordance with Le Chatelier's principle, the equilibrium constant changes to minimize the change in temperature For endothermic reactions, an increase in temperature can be minimized by utilizing some of the heat to convert reactants to products, shifting the equilibrium to the right side of the reaction and increasing the value of K



Solved Use Le Chatelier S Principle To Explain The Change Chegg Com



1 6 Chemical Equilibria And Le Chatelier S Principle Equilibrium As Secondary Science 4 All
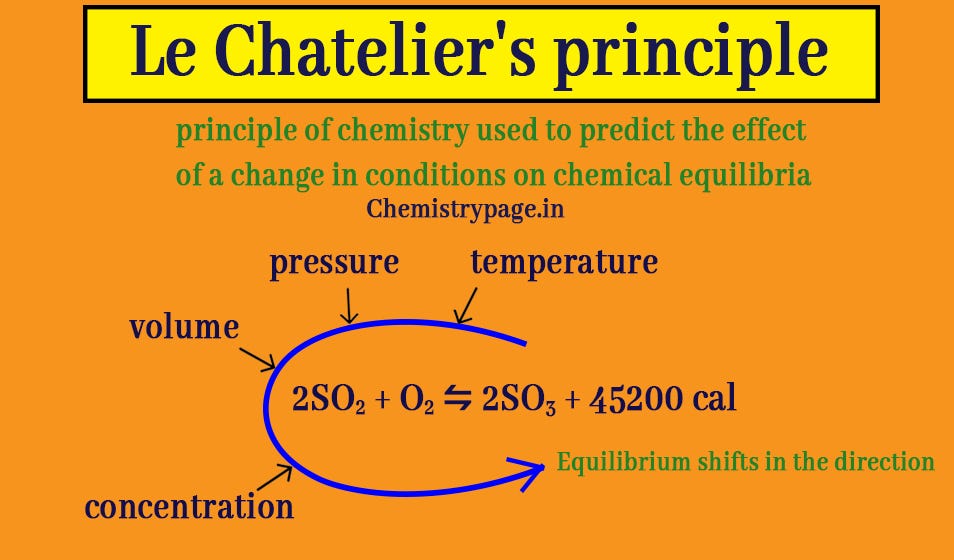
Investigate the effects of concentration, pressure and temperature on equilibrium and explore Le Chatelier's principle in this series of demonstrations Le Chatelier's principle describes how, when a dynamic equilibrium is disrupted by a change of pressure, temperature, volume or concentration, the position of equilibrium will shift to counteract any change and restore an equilibrium state71 Le Chatelier's principle Applications and skills Application of Le Châtelier's principle to predict the qualitative effects of changes of temperature, pIncrease or decrease the temperature ?


Le Chateliers Principle Le Chateliers Principle When A


Le Chatelier S Principle 2
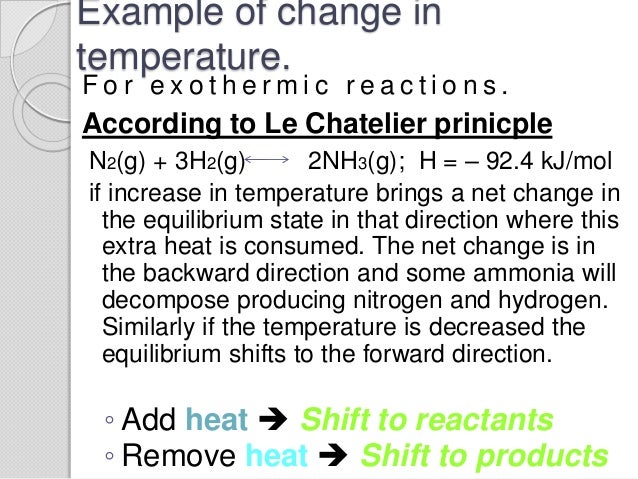
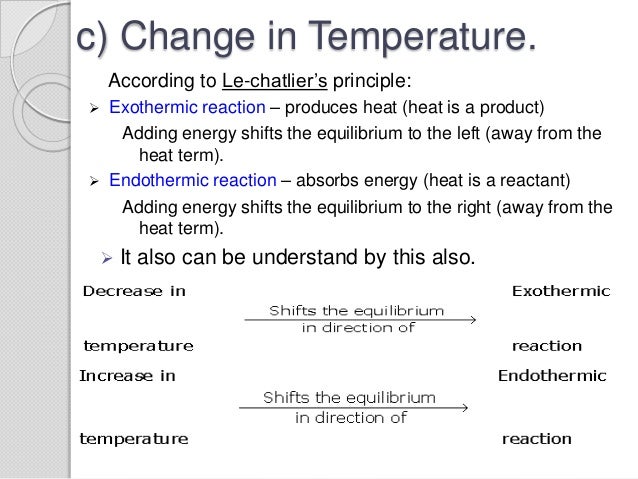
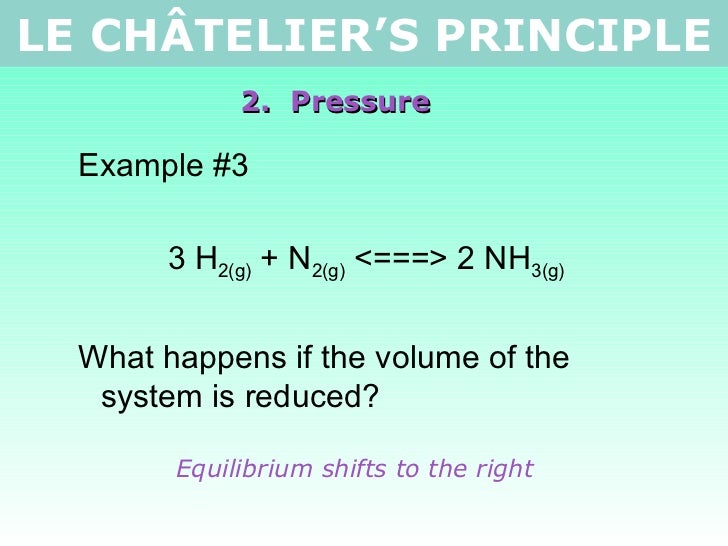
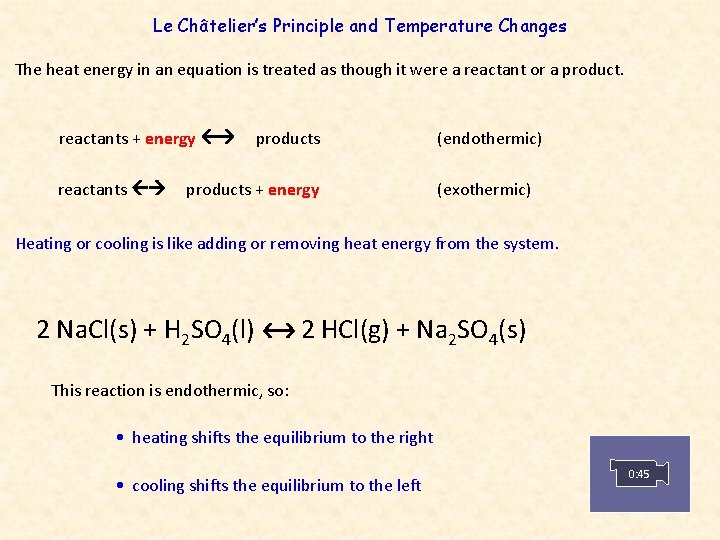
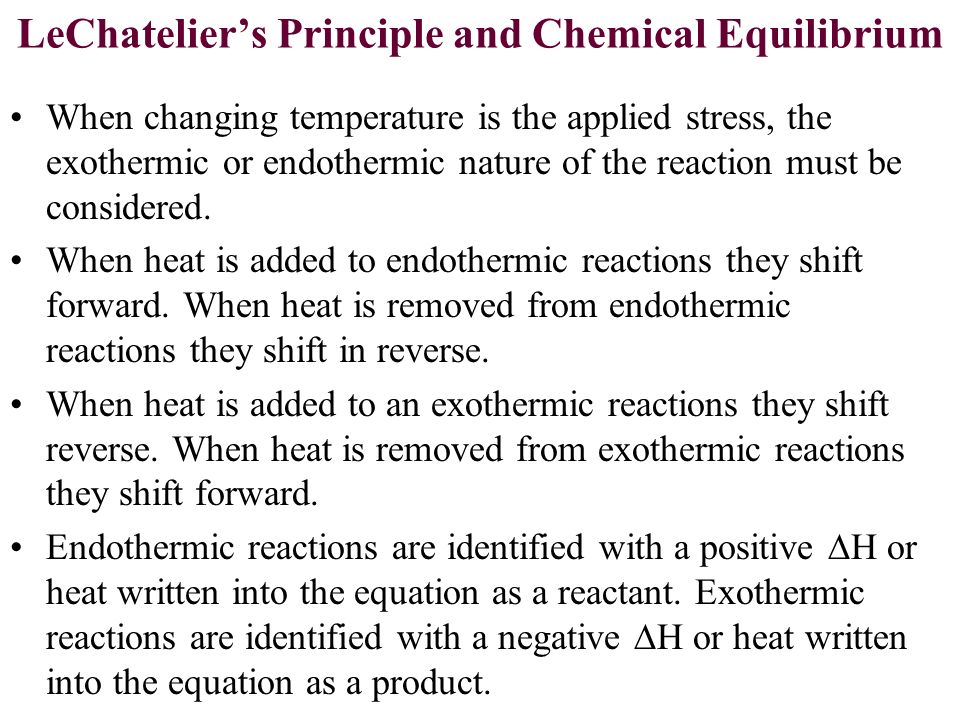
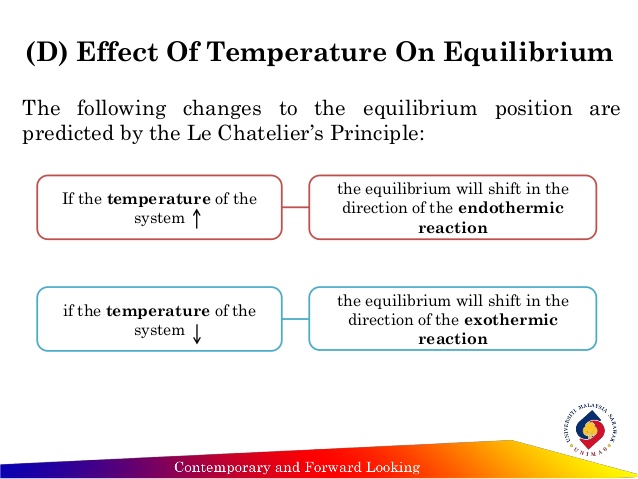
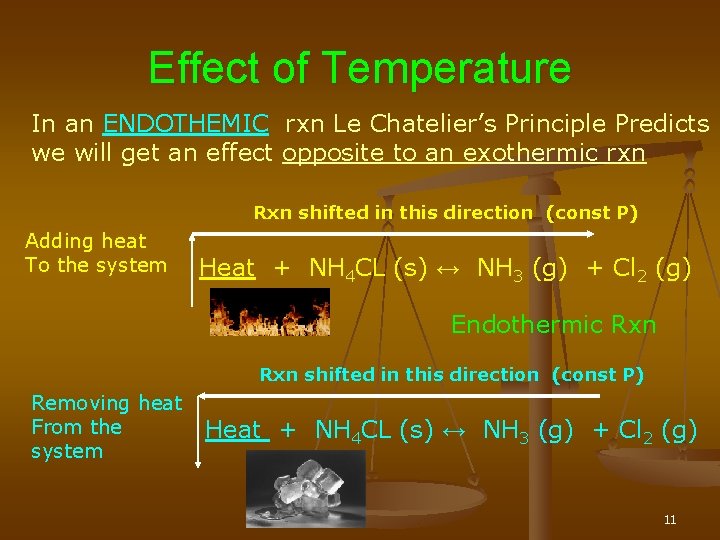
By Le Chatelier's principle, the system will consume some reactants to form products This reduces the forward reaction rate while increases the reverse reaction rate Temperature Figure A rise in temperature causes more particles to have energy greater than the activation energyThe effect of temperature can be understood by using le Chatelier's principle as follows 1) Increase in the temperature of the system favors the endothermic reaction The increase in temperature increases the amount of heat in the systemUsing Le Chatelier's Principle with a change of temperature For this, you need to know whether heat is given out or absorbed during the reaction Assume that our forward reaction is exothermic (heat is evolved) This shows that 250 kJ is evolved (hence the negative sign) when 1 mole of A reacts completely with 2 moles of B


Equilibrium And Le Chatelier S Principle Presentation Chemistry



Lechatelier S Principle And The Equilibrium Constant Ck 12 Foundation
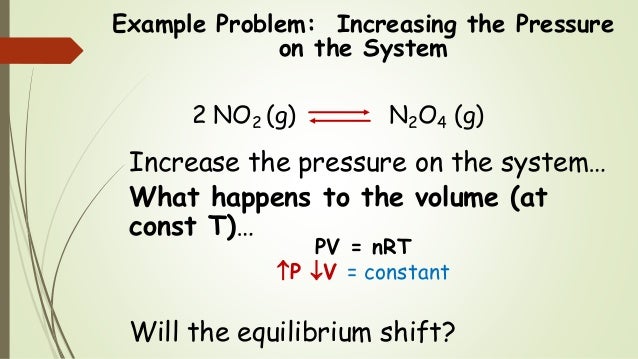
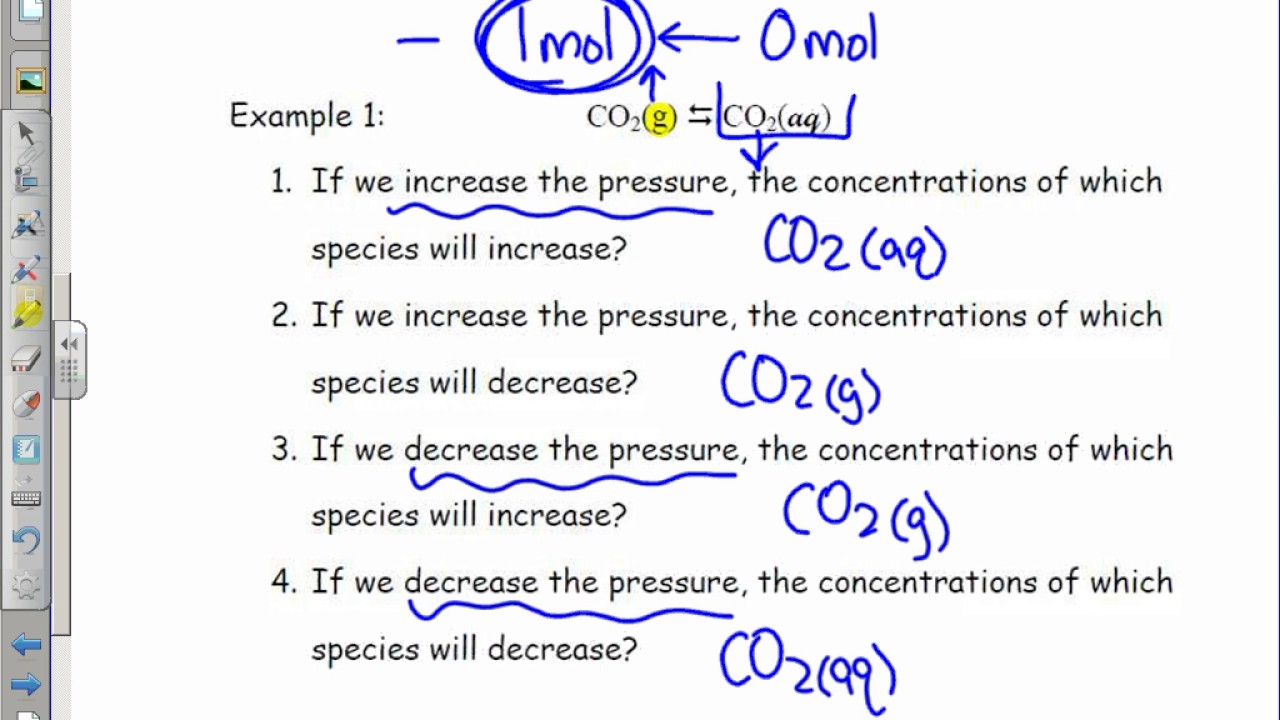
Reducing the volume of the reaction vessel, for example by depressing the plunger of a syringe, while maintaining a constant temperature Immediate impact Concentration of both NO 2 (g) and N 2 O 4 increases Total gas pressure inside reaction vessel increases Application of Le Chatelier's principleLe Châtelier's Principle Page 1 of 15 Properties of Systems in Equilibrium – Le Châtelier's Principle Objectives To perturb chemical reactions at equilibrium and observe how they respond To explain these observations using Le Châtelier's Principle To relate Le Châtelier's Principle to the concept of coupled reactions02/04/ · By LeChatelier's principle, at a constant temperature, increase in pressure will favour a reaction which is accompanied by a decrease in volume and decrease in pressure will favour a reaction which is accompanied by the increase in volume Chemical reactions accompanied by an increase in volume



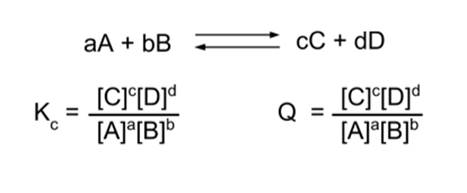
Chemical Equilibrium Dynamic Equilibrium Equilibrium Constant Expression K C K P Q C Le Chatelier S Principle Ppt Download



Hsc Chemistry Le Chatelier Principle And Equilibrium Guide
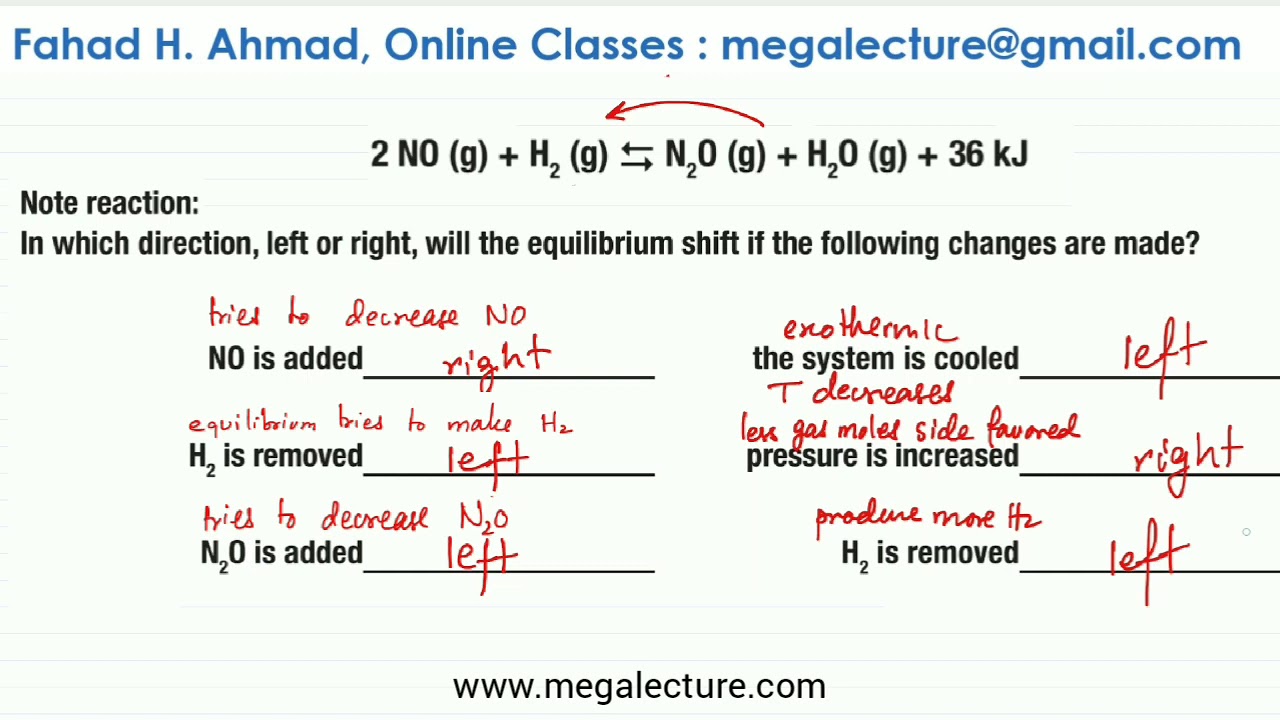
Applications of Le Chatelier's principle definition Effect of temperature on solubility Some solids absorb heat while some evolve heat on dissolution Hence, according to this principle solubility of the former class of solids increases with rise of temperature16/09/14 · Example 7 What is the effect on this equilibrium if pressure is increased?25/12/ · N2(g) O2(g) ⇋ 2NO(g) – 432 kcal According to le chatelier's principle, the effect of concentration, temperature and pressure changes on the equilibrium of this reaction is as follows – Synthesis is increased by increasing the concentration of the reactants Synthesis will be higher at higher temperatures



Reversible Reactions Equilibrium And Le Chatelier S Principle Compound Interest


Chatelier S Principle Presentation Chemistry
13/08/19 · According to Le Chatelier's principle addition of inert gas or noble gas like helium, neon, argon, and krypton is done in two ways as constant temperature and constant volume of the ideal system The addition of inert gas at constant volume can not affect the equilibriumLe Chatelier's Principle Practice Problems for Assignment 4 19 When the temperature of an equilibrium system for the following reaction is increased, CO (g) H Microsoft Word 3Le Chatelierdoc Author Rosamaria Fong Created Date 1/27/08 PMLe Chatelier's principle says that this net reaction will occur in a direction that partially offsets the change The classic example of the practical use of the Le Chatelier principle is the HaberBosch process for the synthesis of ammonia, in which a balance between low temperature and high pressure must be found



Le Chatelier S Principle Worked Example Video Khan Academy


Le Chatelier S Principle 2
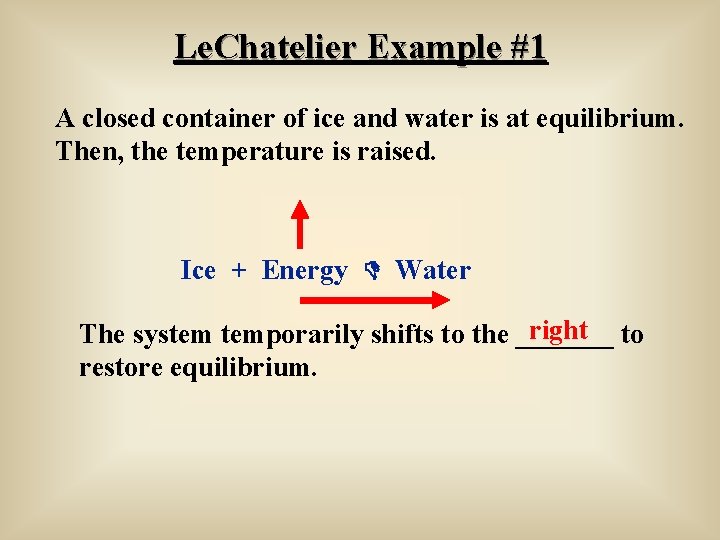
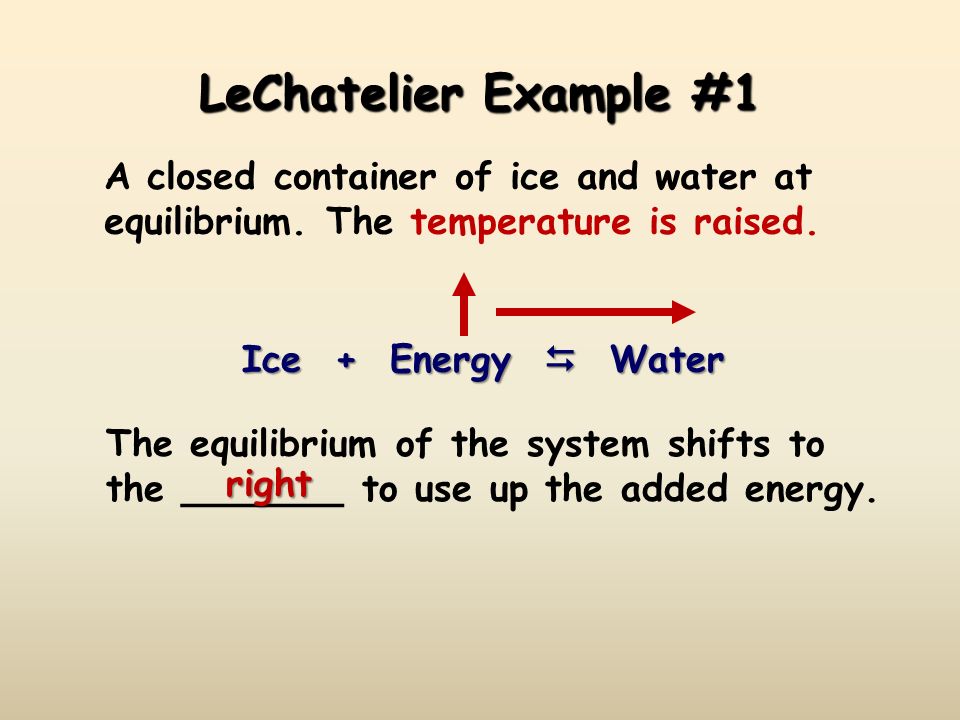
In an endothermic reaction, a decrease in temperature favours the reaction to occur in the backward direction At equilibrium, the concentration of C and D will decrease and the concentration of A and B will increase EXAMPLE H2 I2 HEAT 2HI12/12/14 · Re Le Chatelier's Principle Temperature Post by Vasudev Tadimeti 3B » Tue Dec 09, 14 632 pm If you increase the temperature in an endothermic reaction, the the reaction will favor the products, while in an exothermic reaction, increasing the temperature02/11/19 · Updated November 02, 19 Le Chatelier′s Principle is the principle when a stress is applied to a chemical system at equilibrium, the equilibrium will shift to relieve the stress In other words, it can be used to predict the direction of a chemical reaction in response to a change in conditions of temperature, concentration, volume, or pressure While Le Chatelier's principle



Learning Chemistry Easily Le Chatelier S Principle Effect Of Changing Temperature


The Position Of Equilibrium Topic 7 2 Equilibrium
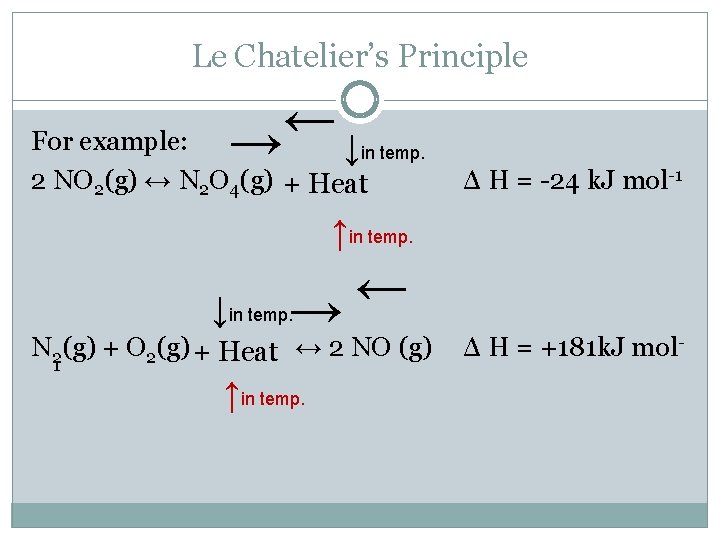
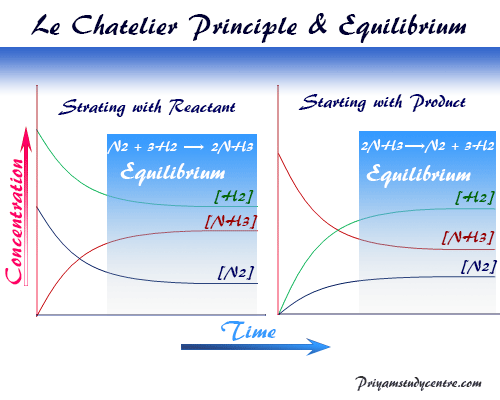
Le Chatelier's Principle In 14 the French chemist and engineer HenryLouis Le Chatelier proposed one of the central concepts of chemical equilibria Le Chatelier's principle can be stated as follows A change in one of the variables that describe a system at equilibrium produces a shift in the position of the equilibrium that counteracts the effect of this change16/11/ · Le Chatelier's principle as related to temperature changes can be illustrated easily be the reaction in which dinitrogen tetroxide is in equilibrium with nitrogen dioxide N 2O 4(g) heat ⇌ 2NO 2(g) Dinitrogen tetroxide (N 2O 4) is colorless, while nitrogen dioxide (NO 2)Le Chatelier's Principle of Chemical Equilibrium This tutorial provides a basic introduction into Le Chatelier's Principle of chemical equilibrium It explains how to determine which direction the reaction will shift if the concentrations of the reactants and products increase in value



Le Chatelier S Principle Ap Chemistry Crash Course Review Albert Io


Le Chatelier S Principle Ppt Download
Increase or decrease the pressure ?Le Châtelier's principle will help us to predict the direction of the equilibrium shift when changes like this are made to the reaction mixtureChanging the temperature When a change is made to a system at equilibrium, the position of equilibrium moves to counteract the change that was made For example, if the temperature



Chemistry Q4 Amazing Benchmark Review Example 1 Standard 9a Know How To Use Le Chatelier S Principle To Predict The Effect Of Changes In Concentration Ppt Download


Chemistry Equilibrium Le Chateliers Principle Temperature And Catalysts
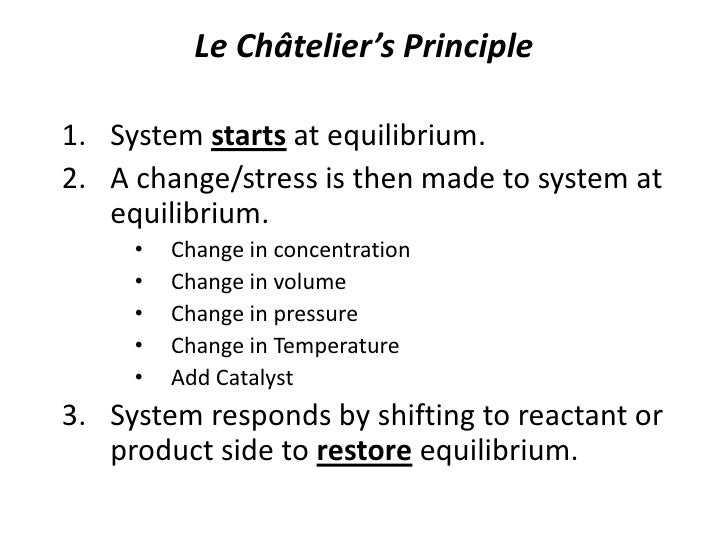
Le Chatelier's principle predicts that the equilibrium will shift to increase the concentration of reactants Increasing the rate of the reverse reaction will mean an increase in reactants So some sulfur trioxide would change back to sulfur dioxide and oxygen to restore equilibrium Equilibrium shifts to the left That is, when a new equilibrium is reached there will be less product than beforeLeChatelier's principle "If a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium moves to partially reverse the change" Gibb's free energy and equilibrium " Equilibrium is attained when the Gibbs free energy of the system is at its minimum value (assuming the reaction is carried out at constant temperature and pressure) "A compromise between a reasonable rate of reaction and a decent yield of product is required in industrial processes The Haber Process is an exothermic reaction in the


Understanding Equilibrium A Delicate Balance Cpd Rsc Education



Le Chatelier S Principle Temperature Problems Youtube
Le Chatelier's principle If a change is made to a reaction that is at equilibrium, the equilibrium will shift in the direction that counteracts the imposed change Add a reactant or product Reaction will consume the added substance Remove a reactant or product Reaction will produce the removed substance Add heat by raising the temperatureOur heat of reaction is positive, so this reaction is endothermic Since this reaction is endothermic, heat is a reactant By Le Chatelier's principle, increasing the temperature will shift the equilibrium to the right, producing more NO 2 Le Chatelier's principle This lesson shows how Le Chatelier's principle predicts changes in an equilibrium It also demonstrates an easy and convenient methodLe Chatelier's Principle Le Chatelier's principle states that when a system in chemical equilibrium is disturbed by a change in temperature, pressure, or concentration, the system will shift in equilibrium composition in a way that tends to counteract this change of variable The three ways that affect this change in equilibrium include



Le Chatelier S Principle Ck 12 Foundation


Pressure Cooker Reduces Cooking Time What Is The Science Behind It By Chemistry Page Medium
The classic example of the practical use of the Le Chatelier principle is the HaberBosch process for the synthesis of ammonia, in which a balance between low temperature and high pressure must be found The previous Module emphasized the dynamic character of equilibrium as expressed by the Law of Mass ActionLe Châtelier's PrincipleChan Sheung ChunThis activity is to let students practice more on the Le Châtelier's PrincipleTo make connections between Le Châtelier's Principle and daily relevant examplesTo apply Le Châtelier's Principle into different situations and predict the outcomesAims12This activity makes use of different scenario Elicit students' understanding about LeA worked example using Le Chatelier's principle to predict how concentrations will shift for different perturbations Example includes changing reaction vessel volume, changing amount of solid product, adding inert gas, and adding a catalyst Created by Yuki Jung Google Classroom Facebook Twitter


Le Chatelier S Principle Vce Chemistry



Le Chatelier S Principle
For example, how is the equilibrium affected if we remove some product D ?08/05/17 · Le Châtelier's Principle states that when we make changes to a reaction at equilibrium, the equilibrium will respond to the change we make to try and undo the change For example, if we increase the temperature of the reaction, it will respond in a way that decreases the temperatureAccording to Le Chatelier's Principle, if you decrease the concentration of C, for example, the position of equilibrium will move to the right to increase the concentration again Note The reason for choosing an equation with "2B" will become clearer when I deal with the effect of pressure further down the page


Tang 02 Le Chatelier S Principle 2



Le Chatelier S Principle Analytical Chemistry Video Clutch Prep
Le Chatelier's principles, also known as the equilibrium law, are used to predict the effect of some changes on a system in chemical equilibrium (such as the change in temperature or pressure) The principle is named after the French chemist Henry Louis Le Chatelier Le Chatelier said that equilibrium adjusts the forward and backward reactions in such a way to accept the changesPractice using Le Chatelier's principle to predict what happens to a reaction when a stress is applied Le Chatelier's principle Worked example Introduction to reaction quotient Qc The reaction quotient Q Comparing Q vs K example Practice Using Le Chatelier's principleLe Chatelier's principle applied to changes in concentration or pressure can be understood by giving K a constant value The effect of temperature on equilibria, however, involves a change in the equilibrium constant The dependence of K on temperature is determined by the sign of Δ H



Chemistry Not Mystery Le Chatelier S Principle Temperature Change


Le Chatelier S Principle
This is a frequent misconception about Le Chatelier's principle Pressure only changes the equillbrium if pressure changes in a way that changes concentrations, for example by changing volume or adding more of a reactant or product


Ppt Catalyst Powerpoint Presentation Free Download Id


Le Chatelier S Principle Worksheet Solved Questions Youtube


What Is Le Chatelier S Principle In Chemistry


Le Chatelier S Principle


According To Le Chteliers Principle When A Chemical



Chemical Equilibrium Haber S Process Definition Examples Diagrams



Solved Experiment 73 Le Chatelier S Principle Online Ob Chegg Com



Chemical Equilibrium The Concept Of Equilibrium No Chemical



What Is Le Chatelier S Principle In Chemistry


Le Chatelier S Principle 2


Factors That Affect Solubility 1 For Solids As


Le Chatelier Principle Definition Application Facts Priyamstudycentre



1 6 Chemical Equilibria And Le Chatelier S Principle Equilibrium As Secondary Science 4 All


What Is Le Chatelier S Principle In Chemistry Socratic



7 1 Le Chatelier S Principle Changes In Temperature Sl Youtube


Question Cc8dd Socratic



Solved Good Morning We Recently Did A Experiment On Chem Chegg Com



Chemical Equilibrium



What Is Le Chatelier S Principle In Chemistry Socratic


Factor Effecting Chemical Equilibrium Fun Science



Le Chatelier S Principle Volume Page 1 Line 17qq Com



Lechatelier S Principle Equilibrium Lechatelier S Principle Co 2 Cao Caco 3 Chicken Breath Food Egg Shell I Wish I Had Sweat Glands As Temperature Ppt Download



Le Chateliers Principle Position Of The Equilibrium Equilibrium



Help Me Please Need It Right Now Thanks In Ad Chegg Com


Le Chatelier S Principle



Using Le Chatelier S Principle To Predict The Result Of Changing Temperature Youtube



Le Chatelier S Principle 2



Factors That Affect Chemical Equilibrium Boundless Chemistry



Lechatelier S Principle Ck 12 Foundation



Chatelier S Principle Page 1 Line 17qq Com



Le Chatelier S Principle And More Ppt Download


What Is Le Chatelier S Principle In Chemistry Socratic


Equilibrium And Le Chatelier S Principle Ppt Download


Tang 02 Le Chatelier S Principle 2



Le Chatelier S Principle Analytical Chemistry Video Clutch Prep


What Is The Le Chatelier S Principle Write The Effect Of Change In Pressure Temperature And Concentration On Equilibrium Quora



7 1 Le Chatelier S Principle Temperature Sl Youtube


Factor Effecting Chemical Equilibrium Fun Science



Comparing Q Vs K Example Video Khan Academy


Shifting Equilibrium The Effect Of Pressure Temperature And


Le Chatelier S Principle Le Chatelier S Principle Is An Idea About How A Reaction Mixture That Is At Equilibrium Will React When It Is Perturbed Away From Equilibrium This Is A General Idea That Can Help Us To Quickly Have Some Insight Into Chemical Equilibria And



Le Chatelier S Principle Pv Changes On Gaseous Sytems Pt 9 Youtube


Chemistry Equilibrium Le Chateliers Principle Temperature And Catalysts



Le Chatelier S Principle Analytical Chemistry Video Clutch Prep


Le Chatelier S Principle



Le Chatelier S Principle Chemistry Video Clutch Prep



Solved 5 Which Are Examples Of Equilibria Which Are Che Chegg Com


Chem 2 Chemical Equilibrium Ix Le Chatelier S Principle And Pressu


Le Chatelier S Principle


Le Chatelier S Principle



Solved Le Chatelier S Principle A Change In Any Of The F Chegg Com



Solved Le Chatelier S Principle States That When Stress I Chegg Com



Ppt Le Chatelier S Principle Powerpoint Presentation Free Download Id



Pdf On Violations Of Le Chatelier S Principle For A Temperature Change In Small Systems Observed For Short Times


Le Chateliers Principle Temperature Example Get Images
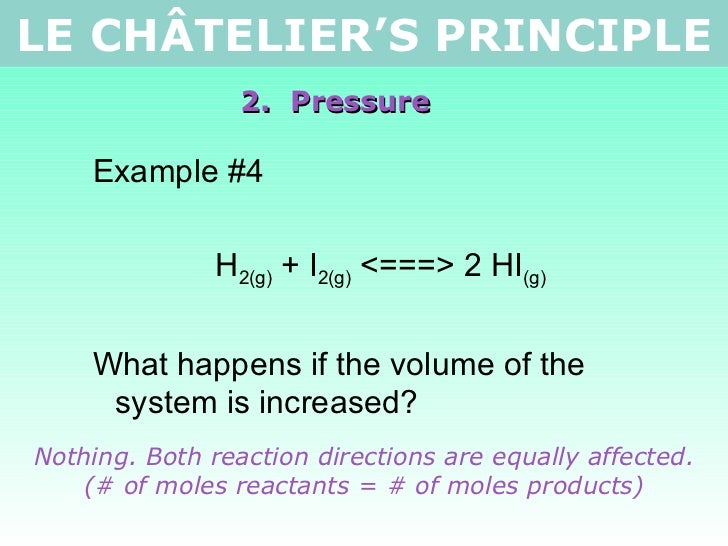


Tang 02 Le Chatelier S Principle 2



7 1 Le Chatelier S Principle Changes In Pressure Sl Youtube
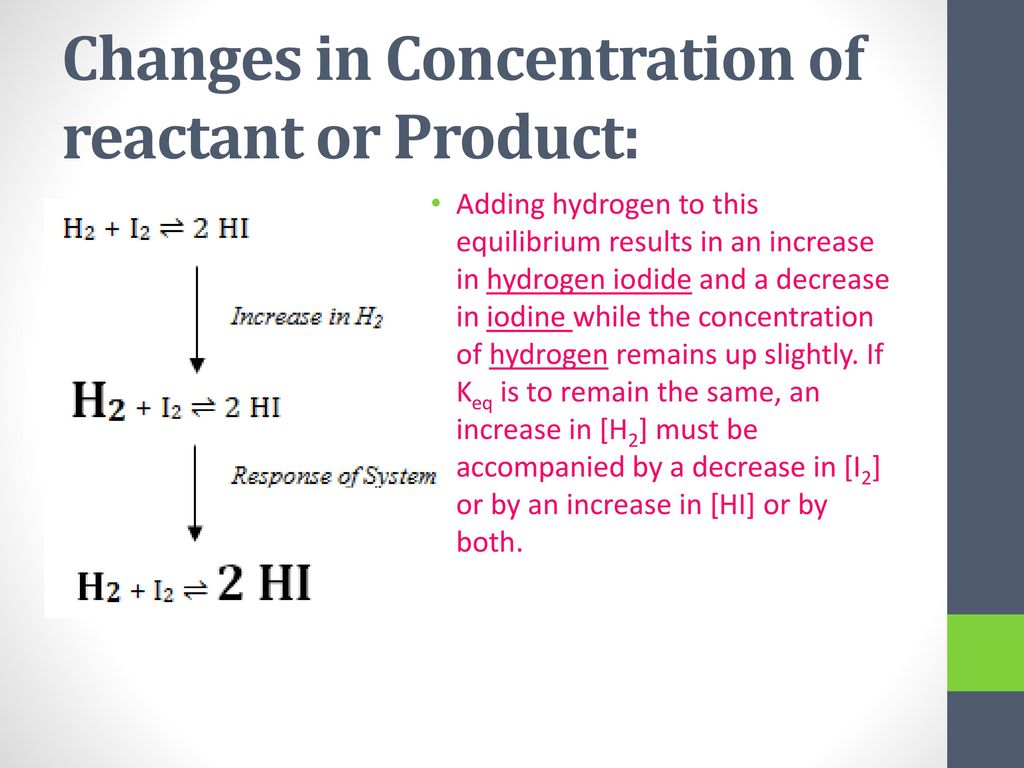


Chem 30 Equilibrium Le Chatelier Ppt Download



Using Le Chatelier S Principle Practice Khan Academy



Solved Exit Fullscreen Le Chatelier S Principle 125 1 Co Chegg Com


Le Chatelier S Principle
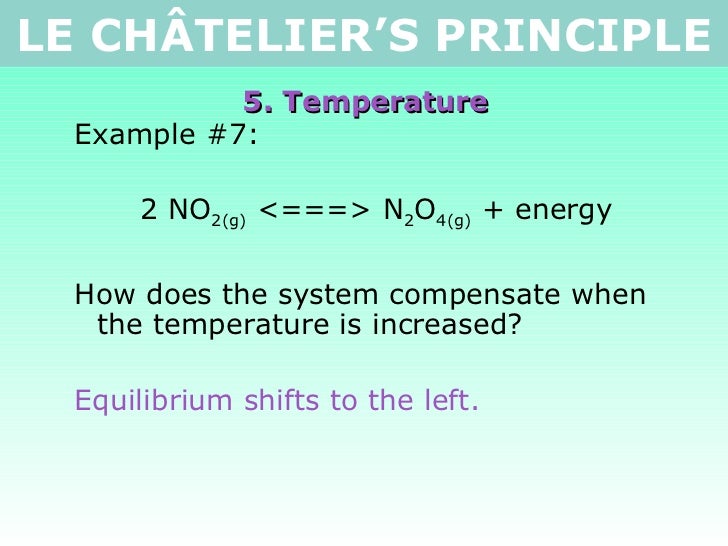


Tang 02 Le Chatelier S Principle 2



Tips For Le Chateliers Principle Concept Chemistry Video By Brightstorm
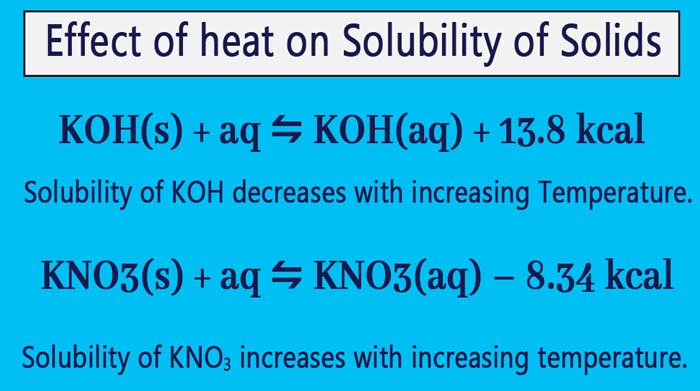


What Is Le Chatelier S Principle In Chemistry


Colloids
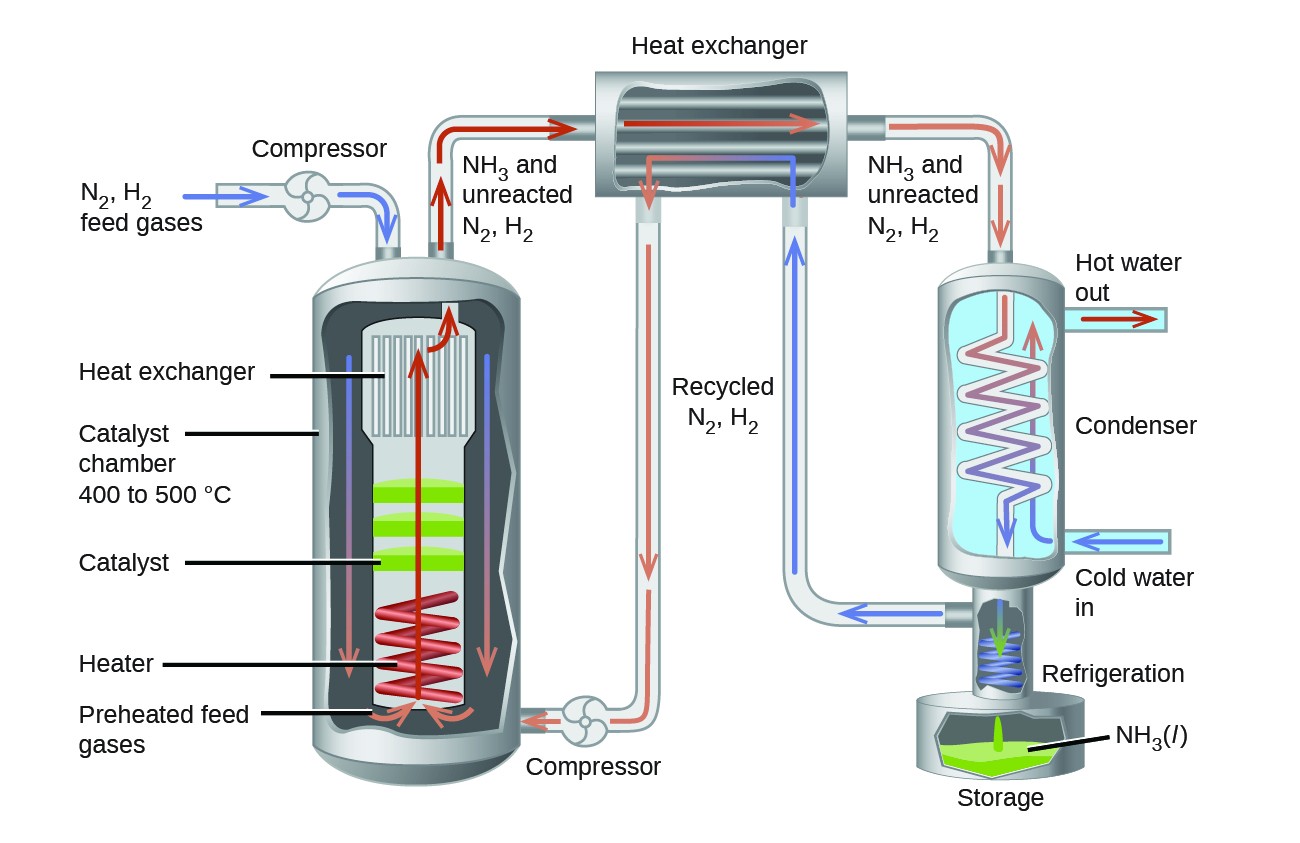


Shifting Equilibria Le Chatelier S Principle Chemistry For Majors



Le Chatelier S Principle



10 Equilibrium Ideas Ap Chemistry Ap Chem Science Chemistry



What Is Le Chatelier S Principle In Chemistry



Aucun commentaire:
Publier un commentaire